Diagnostic Approach in Hematology
Sites of Blood Collection in Different species
|
Animals |
Site of Collection |
|
Horse |
Jugular vein |
|
Cattle, buffalo |
Jugular vein, ear vein |
|
Goat, Sheep |
Jugular vein, ear vein |
|
Pig |
Ear vein, anterior venacava |
|
Dog |
Cephalic vein, lateral saphenous vein, ear vein |
|
Cat |
Ear vein |
|
Fowl |
Wing vein |
Anti-Coagulants used in Blood Collection
Ammonium and Potassium oxalate:
- Proportion of ammonium oxalate to potassium oxalate should be 60:40
- It is known as Heller and Paul’s mixture
- 2 g of ammonium oxalate and 0.8 g of potassium oxalate are taken.
100 ml of distilled water is then added to mixture——-> 0.5 ml of this solution are taken in specimen tube——————> Solution are then incubated at 70°C. this mixture is adequate for 5 ml of blood.
Ethylene diamine tetraacetate (E.D.T.A):
- It may be used as 1 mg powder for 1 ml of blood.
- 1 drop of 10% solution will be adequate for 5 ml of blood.
- It should be not used for biochemical purposes.
Sodium citrate:
- It is prepared by mixing 3.8 g of sodium citrate to 100 ml of distilled water.
- 1 ml of this solution will be required 10 ml of blood.
Sodium fluoride:
- It may be used as 1mg per 1 ml blood.
- It prevents glycolysis and therefore suitable for blood sugar determination.
Heparin:
- It is made as 1% solution
- 1 ml is sufficient for 10 ml of blood.
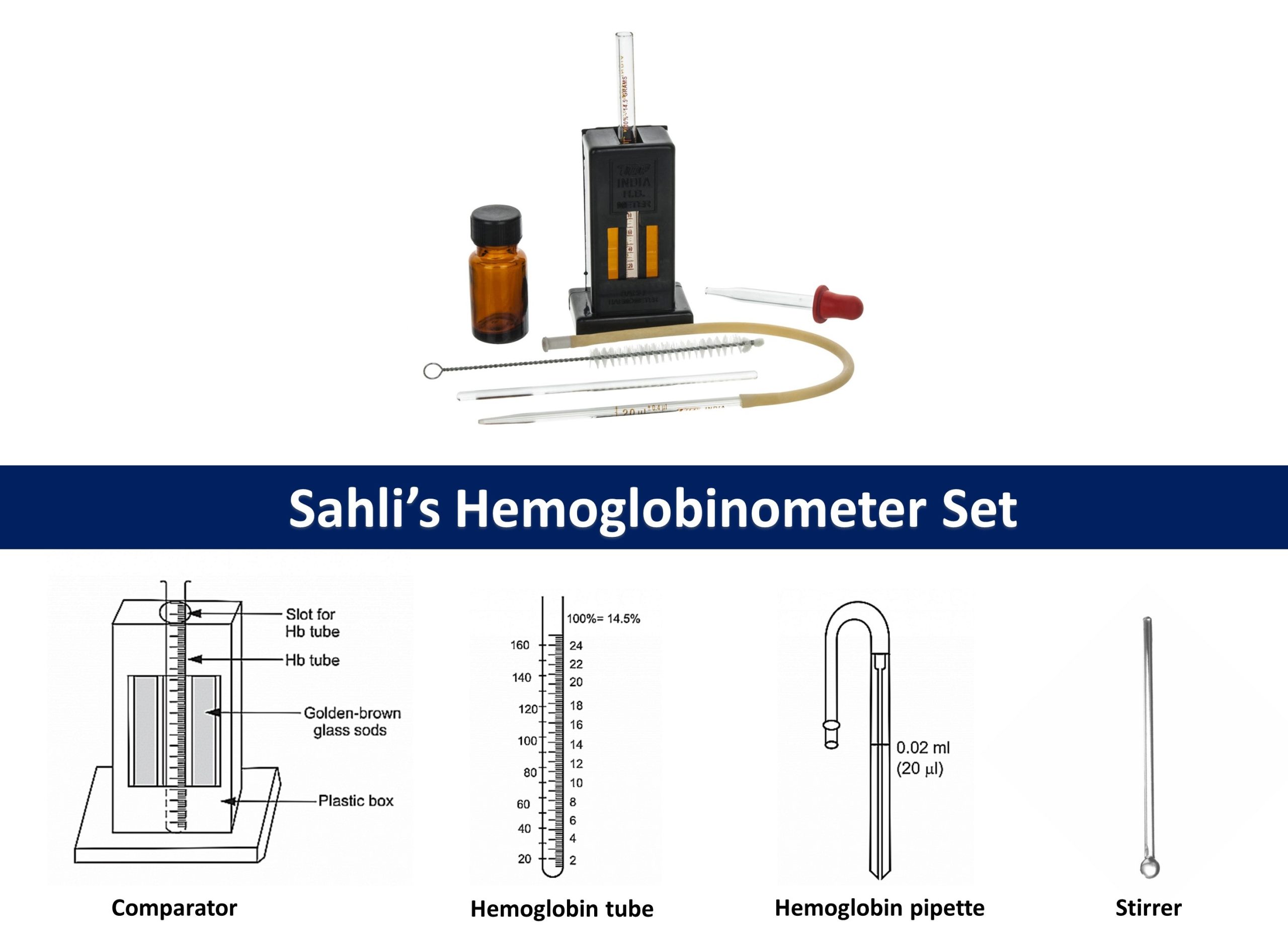
# Determination/Estimation of hemoglobin:
Estimation of hemoglobin through sahli’s hemoglobinometer (Acid-haematin method)
It is visual comparator method for estimation of hemoglobin. As visual comparison method leads to inaccuracy, this method is not used nowadays but was widely used before. the use of spectrophotometric methods like Cyanmethemoglobin method is preferred to it.
Principle:
When the blood is added to dilute hydrochloric acid (HCl), hemoglobin present in the RBCs is converted into brown-colored acid haematin. The acid haematin solution is further diluted until it’s color matches exactly with the permanent standard brown glass compared by direct vision.
Materials Required:
- Sahli’s hemoglobinometer set consisting comparator, hemoglobin tube, hemoglobin pipette and stirrer.
- N/10 hydrochloric acid (HCl)
- Distill water
Procedure:
- Hemoglobinometer tubes and pipettes should be ensured to be dry.
- Hemoglobinometer tube is filled with N/10 HCl upto its lowest mark 2g% or 10% mark with help of dropper.
- 20µL blood is taken in Sahli’s pipette. Extra blood is wiped out and blood is added to hemoglobin tube containing HCl
- Blood and acid are mixed and leaved for about 10 minutes for complete conversion of hemoglobin to hematin
- Distill water is then added drop by drop and stir till the color matches with standard glass of comparator.
- Reading at lower meniscus is then recorded, which directly gives hemoglobin concentration in 100 ml of blood.
Colorimeter method of hemoglobin estimation:
- 20 ml of blood mixed with oxalate or E.D.T.A is drawn with help of hemoglobinometer pipette
- These blood are mixed with 5 ml of Drabkin’s solution (cyanide-ferrocyanide) in test tube.
- After 10 minutes, it is compared with standard with help of colorimeter and recording is recorded.
- It gives value in g per 100 ml of blood.
Spencer Hb-method (Photometer):
- Blood chamber is filled with drop of blood
- Blood is then hemolysed with help of hemolysis stick for 30 seconds.
- Chamber are then inserted in slit on left side of instrument.
- Instruments are hold to look through in left hand. Light switch button is pressed with help of left thumb- green split field will appear in instrument.
- Slide button are then moved on right side until two halves of field are equally light and appear as single field.
- Position of index mark on the slide knot is recorded which indicates hemoglobin concentration.
Normal level of hemoglobin in different animals:
|
Animal |
Hemoglobin level (g/100 ml) |
|
Cattle |
11.3 |
|
Buffalo |
12.9 |
|
Horse |
11.5 |
|
Goat |
10.9 |
|
Sheep |
14.4 |
|
Pig |
11.0 |
|
Dog |
13.0 |
|
Cat |
12.0 |
Decreased in hemoglobin is observed in case of anemia and increased level occurs in polycythemia and hemoconcentration.
# Erythrocyte Count (RBC count)
RBCs are counted using a special chamber designed to count blood cells within specimen. This chamber is called Neubauer’s chamber or Hemocytometer.
Materials Required:
- Hemocytometer or Neubauer’s chamber
- Micropipette
- RBC diluting fluids or Hayem’s fluid
- RBC pipette
- Glass cover
- Blood sample
Procedure:
- First RBC pipette is filled with blood upto 0.5 mark and external part of pipette is wipe out
- Same pipette is then filled with RBC diluting fluid upto 101 mark
- Blood and diluting fluid are then mixed gently by turning pipette horizontally between palm of hands.
- Hemocytometer is then prepared by removing from case and cleaning with soft cotton gauze or swab.
- Clean cover glass is then placed on top of Hemocytometer’s lined region.
- 1-2 drops of blood are discarded during loading Hemocytometer. Rubber of RBC pipette are gently pressed down until fluids are in hanging position. Tip of pipette should touch against edges of coverglass
- Small amount of sample is then placed on edges of coverglass and wait for 3-5 minutes in order to settle down RBC in chamber
- Prepared slide is then observed under microscope to count RBC
Counting of RBC under microscope:
First chamber is focused on 10X. First focus on five small square of central square to count RBC under 40X.

Counting of cell in L-shape of each square is done. Cells touching top, bottom and right side of square is discarded. After counting of cell, number of RBC is then calculated using formula;
Total RBC/µL= Number of RBC counted * Dilution factor/Area * Depth
- Given, dilution factor= 1:200 or 200
- Area; large central square is sub-divided into 25 small or medium squares. So area will be one sq mm. number of RBC counted in 5 small square out of 25 squares. So area will be 5/25 or 1/5 sq mm
- Depth: 0.1 mm
- Total RBCs = N x 200/1/5×0.1 = N x 200 x 50= N x 10,000 cells/µL
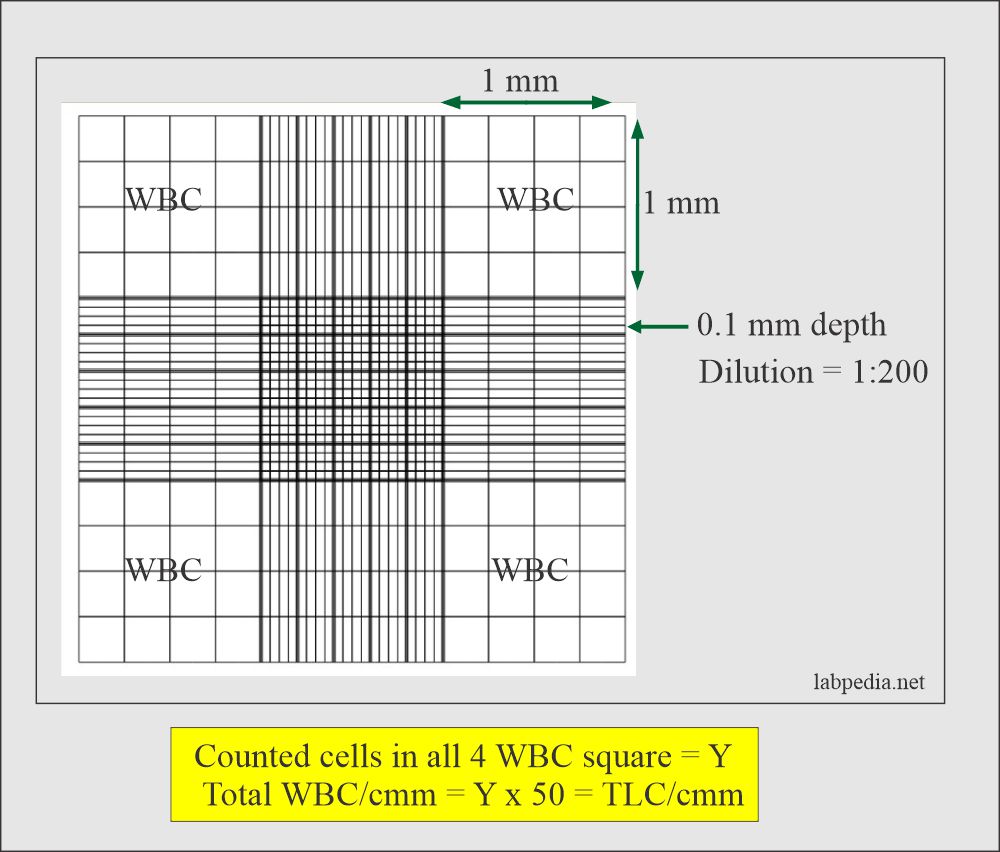
# Leukocyte Count (W.B.C count)
White blood counts determine the amount of white blood cells that are present in animal’s blood. White blood cells form part of animal immune system. They aid body in fighting against infections and other illnesses. WBC count is also done in Hemocytometer or Neubauer’s chamber as in RBC counting.
Materials Required:
- Blood sample (Capillary blood or EDTA anticoagulated specimen)
- WBC diluting fluid (preferably Turk’s fluid)
- Gauze piece or Cotton
- WBC pipette
- Hemocytometer a.k.a. Neubauer’s Chamber
- Coverslip
- Microscope
Procedure:
- First, WBC pipette is filled up to mark 0.5 with blood sample and extra blood outside pipette is wiped out with cotton/tissue paper
- Same pipette is then filled with WBC diluting fluid (glacial acetic acid) upto 11 mark in pipette.
- Blood and then diluting fluid are mixed by rotating pipette horizontally in between palms of hands.
- Neubauer’s chamber is then removed from case and wiped it using clean gauze and swabs.
- Neubauer’s chamber is then loaded with blood through edge of glass slide and leaved for 3-5 minutes to allow to settle down WBC.
- Chambers is then ready to observe under microscope.
Counting WBC under microscope:
- The ruling is focused with the 10x lens and then count the WBCs on four large corner squares in the manner previously described with the 40x objective lens.
- Cells that are located on the lower and right line of 4 Corners are checked and counted in L shape as in RBC

Calculations of WBC
- After counting WBC on 4 square of corners, total number of WBC can be calculated using formula;
Total number of WBC (cells/µL) = Number of WBCs counted x 50
Normal values of RBC and WBC in Animals
|
Animals |
Number of RBC (million/c.mm) |
Number of WBC (Thousand/c.mm) |
|
Cattle |
5.95 |
7.03 |
|
Buffalo |
6.80 |
6.70 |
|
Horse |
6.90 |
5.11 |
|
Goat |
13.90 |
5.14 |
|
Sheep |
8.60 |
4.10 |
|
Pig |
7.40 |
7.30 |
|
Dog |
6.20 |
8.18 |
|
Cat |
7.20 |
9.14 |
# Differential Count of WBC
Differential count of WBC involves determining the concentration of each type of leucocyte. There are 5 different types of leucocytes; neutrophil, eosinophil, basophil, monocyte, lymphocyte. Differential count of WBC is done by preparing a blood smear and staining slide with suitable stain and counting individual number of leucocyte.
Materials Required:
- Glass slide
- Stain; Giemsa stain, Leishman stain or Wright stain
- Microscope
- Hand counter
- Micropipette
- Uncoagulated blood sample
Procedure:
- A drop of blood is placed on clean, grease free slide with help of pipette
- Blood is spreaded with another slide at 45° angle to make uniform smear.
- Glass slides are allowed to dry for some time after formation of smear
- Glass slides are then stained using any one of stain; Leishman stain, giemsa stain
- Staining are done by placing slide on staining rack. Blood smear is covered with Leishman stain for a minute.
- Equal quantity of buffer is added and mixed by blowing. This mixture is allowed to remain on slide for 10 minutes.
- It is then washed in running water. Slides are allowed to dry in air for some time and observed under microscope under 10X. then slides are examined under 100 x under oil immersion
- A corner area is choosen and cells are counted and recorded on hand counter. Cells are counted till 100 cells are observed and percentage of each leukocyte is determined.
Differential leucocyte percentage in different animals
|
|
Cattle |
Buffalo |
Horse |
Goat |
Sheep |
Pig |
Dog |
Cat |
|
Neutrophil |
29.10 |
28.70 |
62 |
40 |
41 |
39 |
62.80 |
44.82 |
|
Eosinophil |
9.87 |
2.90 |
4 |
2.50 |
5.10 |
4.50 |
2-14 |
2-11 |
|
Lymphocyte |
51.40 |
60.60 |
20 |
48 |
50 |
52 |
10-28 |
15-44 |
|
Monocyte |
8.32 |
7.80 |
8 |
2 |
3 |
3.30 |
3-9 |
0.5-0.7 |
|
Basophil |
0-1 |
0.00 |
2 |
1.60 |
0.03 |
1.20 |
0-2 |
0-0.05 |