Measurement of atmospheric pressure
a) Mercury Barometer:
- It is operated with the principle of balancing the column of air against the column of mercury in a sealed glass tube.
- A vertical column of mercury 760 mm long exerts approximately the same pressure (force) per unit area as the atmosphere at sea level.
- The height of the mercury column is proportional to the pressure; P= ρgh
- Where, P= Pressure; ρ =density of mercury (constant); g= acceleration due to gravity (constant); h= height of the mercury column.
- So, P α h

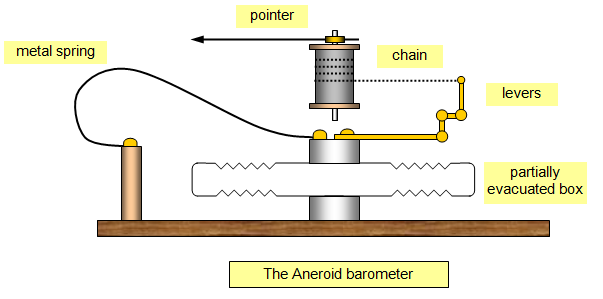
b) Aneroid Barometer:
- A major disadvantage of the mercury barometer is its bulkiness and fragility.
- The long glass tube can break easily, and mercury levels may be difficult to read under unsteady conditions, as on board a ship at sea.
- To resolve these difficulties, the French physicist Lucien Vidie invented the aneroid (“without liquid”) barometer in 1843.
- An aneroid barometer consists of a thin shallow metal box from which all air has been removed.
- Both the top and bottom of the box are corrugated to make them sensitive to changes in the atmospheric pressure.
- When the pressure of the air increases, the box is squashes inward and when the air pressure decreases, the box flexes outward
- A spring is mechanically attached to the box.
- As the box moves in and out in response to the changes in air pressure, the spring expands or contracts & moves the pointer on the dial.
- The dial is calibrated (marked with numbers) so you can read the air pressure instantly.